|
Pauling's taming of the wave equation in his first "Nature of the Chemical Bond" paper
released a flood of new ideas. In June 1931 he submitted a follow-up paper, "The Nature
of the Chemical Bond II. The one-electron bond and the three-electron bond," examining
how quantum mechanics could explain the existence of these relatively rare bonds.
His calculations helped distinguish among alternative explanations for the unusual
bonding properties of oxygen, boron, and nitroso compounds, "odd-electron" molecules
that had deeply interested G. N. Lewis. Lewis himself helped talk Pauling through
some of the ideas in this second paper in what would become a series, the two of them
scribbling sketches and formulae on a blackboard in Lewis's office during Pauling’s
teaching stints at Berkeley, the older man puffing out clouds of cigar smoke and advice.
|
|
Click images to enlarge

"The nature of the chemical bond. II. The one-electron bond and the three-electron
bond." July 11, 1931.
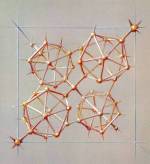
Pastel drawing of Tetragonal Boron. 1964.
"For five years, beginning in spring 1929, I spent one or two months each year in
Berkeley as a visiting lecturer in physics and chemistry. During these extended visits
to Berkeley I had the pleasure of talking with [G.N.] Lewis for many hours, in his
office, his home, and in his Marin County country place."
|

