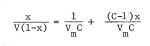When I joined the Fixed Nitrogen Research Laboratory in 1926 I was given the job of learning all I could about the nature of iron catalysts that had been developed for synthesizing ammonia. It was early recognized that no method existed for estimating the surface area of the catalysts with which we were working. Furthermore, it was realized that some method had to be devised for measuring the area of the catalyst particles if one wished to distinguish between activity changes due to alteration of the nature of the surface as compared to changes in the surface extent. We believed that the catalyst particles were almost certainly porous and resembled sponges except they were composed of crystalline iron and had holes about one millionth the size of those in a sponge.
We noted that Dr. A. F. Benton, my former research supervisor at Cal Tech, had measured the adsorption of nitrogen at -195°C on one of the catalysts that we had sent him. He obtained two "kinks" in the plot of volume of nitrogen adsorbed against pressure of the nitrogen. These occurred at nitrogen pressures of 13 and 48 cms of mercury, respectively. He suggested that these kinks might represent the formation of one and two layer of adsorbed nitrogen, respectively. Although we discovered that both "kinks" disappeared when we corrected for the imperfection of nitrogen gas near its boiling point, we concluded that the suggestion was a good one.
In collaboration with Dr. Stephen Brunauer, my assistant, a number of isotherms of nitrogen and other gases were measured near their boiling points. We selected a point (called point B) on the adsorption isotherms that we believed corresponded to a monolayer of adsorbed nitrogen molecules. A simple multiplication of the number of molecules in the monolayer by the area occupied by each molecule then yielded us values for the surface area of the catalyst. These turned out to be in the range 1 to 15 square meters per gram.
Dr. Brunauer then collaborated with Dr. Edward Teller, a new arrival in the Physics Department at George Washington University, in deriving what has become known as the BET equation:
where V is the volume of gas adsorbed at a relative pressure x, Vm is the volume of gas required to form a monolayer and C is a constant. The equation was applied to our experimental data and published formally in 1938 by Brunauer, Emmett and Teller as the BET method for measuring surface areas of finely divided or porous solids. The method proved to be popular and is still used today throughout the world in surface work. Incidentally the values for Vm have turned out to be in good agreement with the "point B" initially suggested as a monolayer.
Other contributions to the understanding of ammonia synthesis process and catalysts were made by our group at the Fixed Nitrogen Research Laboratory during this 11 year period (1926-37). These included measurements of the rates of nitrogen adsorption by the iron catalysts at various temperatures and pressures. We concluded that the slow step in ammonia synthesis was the adsorption of nitrogen on the catalyst surface—a conclusion that is still generally accepted. A new method was worked out for measuring the extent of surface coverage of the iron catalysts by the Al2O3 and K2O-Al2O3 promoters that are deliberately added to the iron catalyst in one to three percent quantities. The method involved measuring the extent of chemisorption of carbon monoxide on the iron portion of the catalyst surface. The method is basically the same as that used today in measuring the extent of surface of the metallic portion of supported metallic catalysts.
The ammonia catalysts were found to be about 60% covered by the few percent promoters present. The free energies of formation of the nitrides Fe4N, Fe3N and Fe2N were determined for the first time. It is now clear that these nitrides cannot possibly be serving as intermediates in the ammonia synthesis. It was found that two different types of hydrogen chemisorption occurred on the doubly promoted catalysts in the temperature range -195° to 450°C; on the singly promoted catalyst containing only Al2O3 as a promoter three types of hydrogen chemisorption were shown to exist. All in all, the 11 year period spent at the Fixed Nitrogen Research Laboratory saw the completion of extensive basic research on the factors influencing the activity of the iron ammonia catalysts. It may fairly be said to have laid the foundation for much of the later work that has followed.
One other interesting and unusual set of experiments at the Fixed Nitrogen Research Laboratory should perhaps be mentioned. One of the important reactions used in preparing the hydrogen for ammonia synthesis is the water gas shift reaction
H2O + CO = CO2 + H2
In the early 1930's two values for the equilibrium constant for this reaction existed differing from each other by about 40%. No explanation for the discrepancy was known. Since equilibrium considerations are usually important to any chemical process it became necessary for us to find the correct value for the equilibrium constant and if possible to show the reason for the 40% discrepancies. A review of the literature revealed that all constants obtained by letting a mixture of CO, H2O, H2 and CO2 equilibrate over a catalyst showed fairly good agreement among the various workers. However an equally good value should be obtained by measuring the equilibrium constants for the two reactions
FeO + H2 = H2O + Fe
and Fe + CO2 = CO + FeO
and then combining the two to yield the equilibrium constant for the water gas shift reaction. A literature survey showed that these indirect determinations differed among themselves by as much as 40%. The difficulty apparently was to be found in determining equilibrium values for reaction 2. A forty percent spread in the values of the equilibrium constant for this reaction was found to exist. So we set up an apparatus designed to unravel the cause for the discrepancies. We succeeded in showing that the error in some of the values reported for reaction 2 were caused by a phenomenon known as thermal diffusion. Without going into the gruesome details of the theory of thermal diffusion, one can illustrate it by pointing out that a mixture of gases of different molecular weights—as for example a mixture of helium and carbon dioxide—will attain a different composition in a portion of the container tube heated to a high temperature than in a portion at room temperature. For water vapor and hydrogen mixtures this error can amount to as much as 40% and explains the discrepancies in the published results for the water gas shift reaction. This was, I believe, the first time that this thermal diffusion phenomenon had been used to explain discrepancies in equilibrium constants.